The Power of CAPA: Enhancing Operational Effectiveness
Introduction
In the dynamic and complex landscape of business, operations require flexibility and adaptability to various situations. Without having a CAPA process in place, such situations can lead to risky behaviors. An example of this is seen in a food company’s response to an abrupt surge in customer demand. To address this unexpected upswing, the company temporarily adopted a strategy known as “quality release in transit.” However, what began as a one-time exception soon evolved into a habitual practice for addressing unforeseen spikes in demand. The company’s quality control systems were not tuned to meticulously track the quality status of every shipment dispatched. This oversight materialized into a genuine concern when a specific batch was flagged for quality review, but by that time, it had already been dispatched to customers.
The above instance shows how shortcuts become rules and exceptions become norms, if the appropriate actions are not taken on time. This is precisely why CAPA, or Corrective Actions and Preventive Actions, are essential to ensure that operations stay on the right course. It is a systematic approach aimed at identifying, addressing, and preventing issues within a business process. It involves a structured process of investigation, root cause analysis, corrective action, and preventive measures. The primary goal of CAPA is to enhance overall efficiency, minimize errors, and ensure continual improvement.
In the Food Company example mentioned earlier, had the exceptional release been flagged and addressed promptly with the application of CAPA, the incident could have been averted.
Importance and Benefits of Corrective & preventive Actions
The role of the corrective and preventive action subsystem is to gather, evaluate, and address data related to process, product and quality issues. This involves identifying and delving into problems, implementing suitable corrective and/or preventive measures, and ensuring that these issues do not resurface. Crucial steps include verifying or validating these actions, sharing the progress with accountable individuals, supplying pertinent details for management reviews, and maintaining thorough documentation. Effectively managing product and quality challenges, preventing their reoccurrence, and minimizing device failures heavily rely on these activities. Undoubtedly, the corrective and preventive action subsystem stands as one of the most pivotal components within a quality system.
Implementing CAPA effectively yields a plethora of benefits that contribute to the growth and success of an organization:
Improved Efficiency: CAPA fosters a culture of proactive problem-solving, leading to swift issue resolution and minimal disruption to operations.
Enhanced Compliance: Regulatory compliance is a paramount concern for many industries. CAPA ensures adherence to regulations by rectifying non-conformities promptly.
Reduced Risks: By identifying and addressing potential risks early on, CAPA mitigates the likelihood of errors, accidents, and costly setbacks.
Cost Savings: Addressing issues at their root cause prevents recurring problems, saving both time and resources.
Innovation: As you dissect and address the issues, organizations gain valuable insights that can fuel innovation and process optimization.
CAPA Process
The CAPA process should be well-structured & comprehensive, yet it has to be nimble enough for faster implementation. There is a school of thought that recommends 10-15 steps in CAPA process involving approvals at various stages. While it may be relevant for large scale initiatives but in majority of cases it should not require so many steps. People responsible for the operations have to be skilled & trained in CAPA to be able to take responsibility for its implementation without getting involved in too much paperwork & bureaucracy.
A typical CAPA Process should consist of following steps:
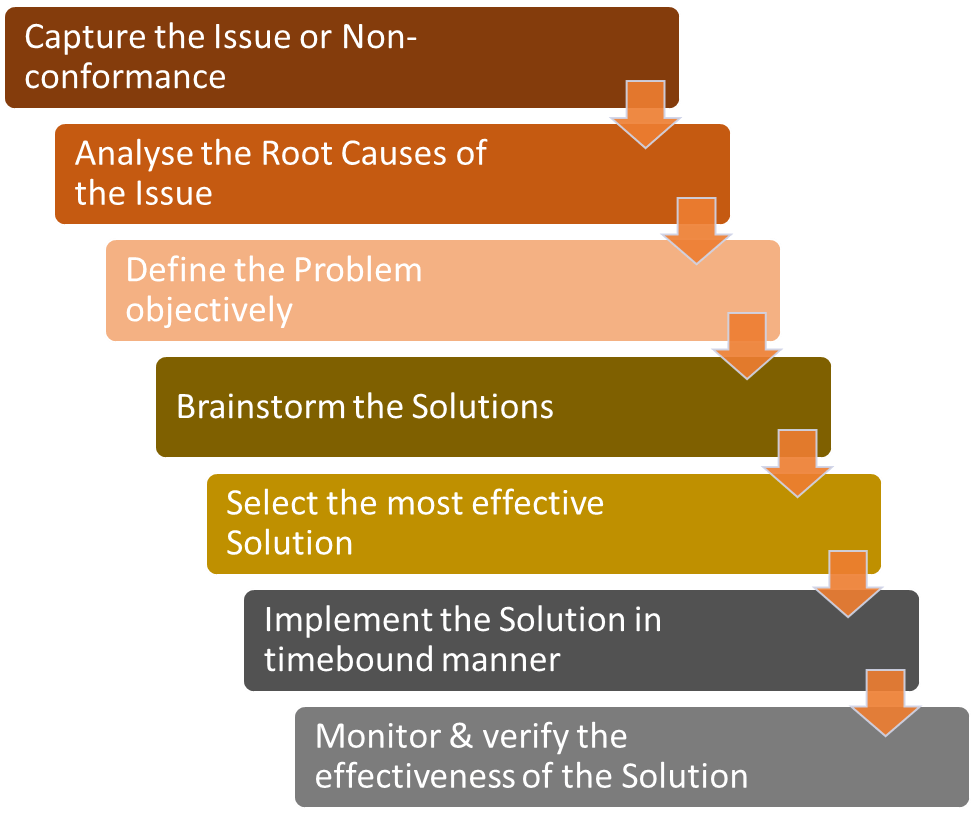 The process involves application of various problem-solving techniques e.g. 5 Whys to define the problem accurately, data analysis and Ishikawa (fishbone) diagram for the root cause analysis, brainstorming etc.
The process involves application of various problem-solving techniques e.g. 5 Whys to define the problem accurately, data analysis and Ishikawa (fishbone) diagram for the root cause analysis, brainstorming etc.
However, in a majority of cases, the solution may be straight forward e.g., retraining people on certain violations. The idea is to have a right balance between thoroughness and agility. Also, CAPA should not require another department or set of people to carry out the process. What truly matters is the competence and training of the individuals overseeing operations. Equipping them with CAPA skills empowers them to shoulder the responsibility of its execution effectively.
Best Practices for Implementing CAPA
To harness the full potential of CAPA and reap its benefits, organizations should follow these best practices:
Clear Process Documentation: Document the entire CAPA process, including the steps from issue identification to root cause analysis, corrective actions, and preventive measures. This ensures consistency and transparency.
Cross-Functional Collaboration: Encourage collaboration among departments and teams to obtain diverse perspectives and insights into the issues at hand.
Root Cause Analysis: Devote adequate time to identifying the root causes of problems rather than just addressing the symptoms. Tools like Fishbone Diagrams and 5 Whys can aid in this process.
SMART Goals: Set Specific, Measurable, Achievable, Relevant, and Time-bound (SMART) goals for corrective and preventive actions. This helps in tracking progress and evaluating the effectiveness of the measures taken.
Regular Review and Analysis: Continuously monitor and analyze the effectiveness of implemented CAPA processes. Adjust them as needed based on outcomes and feedback.
Employee Training: Provide training to employees on CAPA procedures and methodologies. Empower them to contribute to problem-solving and suggest preventive measures.
Data-Driven Approach: Base decisions on data and evidence rather than assumptions. Collect relevant data to support root cause analysis and measure the impact of corrective actions.
Use Technology: Tracking of CAPA across various functional areas for timely & effective implementation can be a nightmare for the operational leaders. Use of IT tools like SIMSA, that not only help to identify the gaps but also enable the tracking of CAPA with the help of auto reminders to the people responsible for each CAPA.
Continuous Improvement Culture: Integrate CAPA into your organization’s culture. Encourage employees to report issues without fear of reprisal and reward innovation and proactive problem-solving.
Do’s and Don’ts of CAPA Implementation
To ensure a successful CAPA implementation, adhere to these do’s and avoid the corresponding don’ts:
Do’s:
Foster Accountability: Assign clear responsibilities for each step of the CAPA process to ensure ownership and accountability.
Communicate Effectively: Maintain transparent communication channels to keep stakeholders informed about the progress of CAPA initiatives.
Prioritize High-Impact Issues: Focus on issues that have the most significant impact on operations, customer satisfaction, and regulatory compliance.
Benchmark and Learn: Study successful CAPA implementations in similar industries to gain insights and adapt best practices to your organization.
Track CAPA: Put a solution in place to track the CAPA implementation against the agreed timelines. Automate the tracking process.
Celebrate Successes: Recognize and celebrate successful CAPA outcomes to motivate employees and reinforce the importance of the process.
Don’ts:
Blame Individuals: Avoid pointing fingers at individuals for issues. Instead, concentrate on identifying systemic causes and implementing corrective measures.
Rush the Process: Rushing through the CAPA process can lead to incomplete root cause analysis and ineffective solutions.
Neglect Documentation: Inadequate documentation can hinder the tracking of progress, evaluation of results, and compliance with regulatory requirements.
Overlook Preventive Actions: Emphasize preventive actions as much as corrective actions to avoid recurring problems.
Fear Change: Be open to altering existing processes and approaches to align with the insights gained from CAPA implementation.
Conclusion
Corrective Actions and Preventive Actions stand as a beacon of operational excellence, offering organizations a roadmap to continuous improvement, risk mitigation, and enhanced efficiency. By following the best practices outlined in this article and adhering to the do’s and don’ts, organizations can embrace CAPA as a transformative tool that fosters growth, innovation, and success in today’s dynamic business landscape. Harness the power of CAPA to unlock a future of streamlined operations, reduced risks, and satisfied customers.



