How to Implement Lean Warehousing
Introduction
Lean warehousing is an adaptation of the Toyota Production System, which focuses on optimizing efficiency, reducing waste, and continuously improving processes. Originating from Toyota’s manufacturing practices, lean principles have been successfully applied to various industries, including warehousing, to enhance overall operations and performance. Implementing a lean warehouse operation can significantly reduce waste, improve efficiency, and increase overall productivity. A lean warehouse can reduce cost by 40%, improve throughput by 25%, and reduce errors by 80%. Lean warehouse management exposes the hidden bad practices & inefficient ways of working, which can be addressed once visible to everyone. There are many other benefits e.g. shorter cycle times, reduced vehicles TAT, improved customer service and so on. Here’s a guide on how to get started.
Understanding Lean Warehousing
Lean warehousing is a strategic approach to warehouse management that focuses on eliminating waste and maximizing efficiency. Inspired by lean manufacturing principles, it seeks to optimize processes, reduce inventory levels, and improve overall productivity. By identifying and eliminating non-value-added activities, lean warehousing creates a more agile and responsive warehouse operation. At the heart of lean warehousing are the following principles:
- Value: Identify what customers value and focus on activities that add value to the customer. By focusing on customer value, you ensure that all activities in the warehouse contribute to meeting customer expectations, reducing unnecessary steps and enhancing overall efficiency.
- Value Stream: Mapping the value stream allows you to identify and eliminate waste, such as excessive movement, waiting times, and overprocessing. This leads to a streamlined process that is quicker and more cost-effective.
- Flow: Establishing a smooth flow ensures that products move efficiently through the warehouse, minimizing delays and reducing the time it takes to process orders. This improves turnaround times and enhances customer satisfaction.
- Pull: Adopting a pull system aligns inventory levels with actual demand, reducing excess stock and minimizing storage costs. This ensures that resources are used effectively and that products are available when needed.
- Perfection: Continuous improvement drives ongoing enhancements in warehouse operations. By regularly assessing and refining processes, you can eliminate errors, improve accuracy, and increase overall productivity.
When these principles are applied in a systematic way to warehousing operations, it paves the way for a host of improvement initiatives that add to the overall supply chain efficiency & responsiveness.
Key Benefits of Lean Warehousing
Implementing lean warehousing practices can yield significant benefits:
- Reduced Costs:
By eliminating waste and optimizing processes, businesses can achieve substantial cost savings. Lean warehousing helps to cut down on unnecessary activities, reduce excess inventory, and streamline workflows, which collectively lower operational expenses. This efficiency leads to a more cost-effective warehouse operation, enabling businesses to allocate resources more strategically.
- Improved Efficiency:
Streamlined operations lead to faster order fulfillment, reduced lead times, and increased productivity. Lean principles focus on creating smooth workflows and minimizing bottlenecks, ensuring that each step in the warehousing process is efficient and value-driven. This results in quicker processing times and the ability to handle more orders without compromising on quality.
- Enhanced Customer Satisfaction:
Higher levels of service and on-time delivery contribute to improved customer satisfaction. Lean warehousing practices ensure that products are picked, packed, and shipped accurately and promptly. This reliability boosts customer trust and loyalty, as they receive their orders correctly and on schedule, leading to repeat business and positive word-of-mouth.
- Increased Flexibility:
A lean warehouse is better equipped to adapt to changing market conditions and customer demands. Lean principles emphasize flexibility and responsiveness, allowing warehouses to quickly adjust to fluctuations in order volumes, seasonal demands, and other market dynamics. This adaptability ensures that the warehouse can meet customer needs effectively, even in a rapidly changing environment.
- Better Space Utilization:
Optimized storage and layout maximize the use of available space. Lean warehousing involves carefully planning the warehouse layout to reduce wasted space and improve accessibility. Techniques such as vertical storage, efficient shelving, and strategic placement of high-turnover items help in making the most of the available space. This not only increases storage capacity but also improves overall workflow and efficiency.
How to Implement Lean Warehousing
Transforming a warehouse into a lean operation requires a systematic and strategic approach. Here’s a step-by-step guide to help you implement lean warehousing effectively:
1. Define Lean Objectives:
2. Mapping the Value Stream:

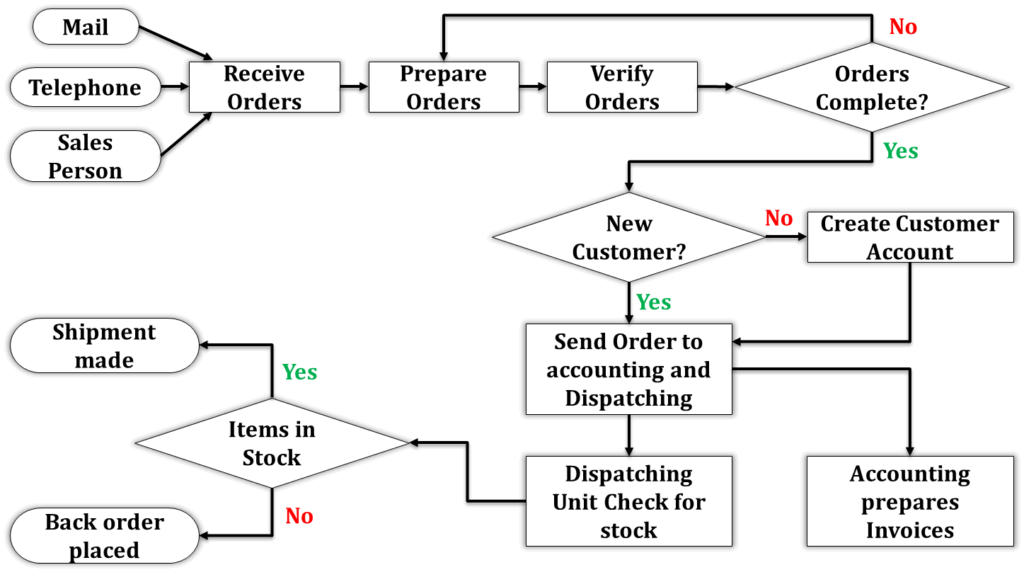
Mapping the value stream is a crucial step in implementing lean warehousing. This process involves documenting every step involved in your warehousing operations, from receiving goods to shipping them out. The goal is to create a visual representation of the entire workflow, which helps identify areas of waste and opportunities for improvement. Here’s how to effectively map the value stream:
- Identify Key Processes : Start by identifying all key processes in your warehouse. This includes receiving, storage, picking, packing, and shipping. Each process should be clearly defined and broken down into individual steps.
- Gather Data: Collect detailed data on each step of the process. This includes time taken for each task, resources used, inventory levels, and any delays or bottlenecks. Use direct observation, interviews with staff, and existing records to gather accurate information.
- Create a Flow Diagram: Create a flow diagram that visually represents the sequence of steps in your warehousing processes. Use standard symbols to represent different types of activities (e.g., processing, transportation, waiting) and connect them with arrows to show the flow of materials and information.

3. Identify Wastes or Non-Value Adding Activities:
Distinguish between value-added activities (those that directly contribute to meeting customer needs) and non-value-added activities (those that do not add value and may be considered waste). In lean philosophy, there are 8 types of wastes in any operation. These are:
 When these wastes are applied to warehousing operations, it would imply the following:
When these wastes are applied to warehousing operations, it would imply the following:
Defects: Errors in transaction, mix-up of SKUs or Batches, storage at wrong locations, documentation errors, inaccurate inventory records etc.
Overproduction: Excess picking and packing, generating more invoices than that can be shipped, splitting a shipment into multiple invoices etc.
Waiting: Vehicles waiting for loading / unloading, putaway waiting for inspection or availability of space or equipment, pickers waiting for the picklist generation etc.
Transportation & Motion: Long distances traveled to retrieve or store items, backtracking during order picking, or unnecessary movement of empty pallets, reaching for items on high shelves, or bending and lifting to access products etc.
Inventory: Overstocking items more than the demand, defective items, slow-moving & obsolete items occupying prime storage locations.
Overprocessing: Excessive paperwork, redundant checks, or unnecessary packaging.
Unutilized Talent: Not involving employees in problem-solving, lack of cross-training, or failure to recognize employee suggestions.
By identifying and eliminating these wastes, warehouses can significantly improve efficiency, reduce costs, and enhance customer satisfaction. It’s essential to involve employees in the process, as they often have valuable insights into waste reduction opportunities.
4. Make Plans to Eliminate Wastes
Once you have identified the wastes and assessed their impact on the process outcomes, the next step is to make plans for eliminating wastes. The plan should prioritize the wastes that have a greater impact on the objectives identified in the step 1. Here are some strategies to address the eight wastes of lean in a warehouse setting:
Overproduction: While the term “overproduction” might seem less applicable to warehouses compared to manufacturing, it’s still a critical concept. In a warehousing context, production can be interpreted as picking, packing, documentation. The strategies for eliminating over production are:
- Demand-driven planning: Use actual orders (instead of a forecast) to align production with actual customer needs.
- Kanban system: Implement a pull system to produce or order items only when needed.
- Level scheduling: Distribute production evenly to avoid peaks and valleys.
Waiting
- Line balancing: Optimize workflow to eliminate idle time.
- Continuous Workflow: Reduce the batch size of each activity or best use the automation for continuous flow e.g. using conveyors, sorters, packing lines.
- Automate Tasks: Automation like pick to light, scanners, AS/RS can reduce the search time, storage, retrieval and recording of transaction.
- Preventive maintenance: Reduce equipment breakdowns.
- Cross-training: Develop versatile employees to cover multiple tasks.
Transportation
- Optimized layout: Design the warehouse layout to minimize material movement. Storing fast moving products near the docks and at the ground level can help to minimize transportation.
- Material handling equipment: Invest in efficient equipment.
- Load optimization: Maximize load capacity to reduce trips.
Inventory
- ABC analysis: Categorize inventory based on value and turnover to focus on critical items.
- Just-in-time (JIT) inventory: Reduce inventory levels by coordinating with suppliers.
- Cycle counting: Improve inventory accuracy.
Motion
- Ergonomic workstations: Design workspaces to minimize employee movement.
- 5S methodology: Create a clean and organized workspace.
- Visual management: Use visual aids to locate items quickly.
Overprocessing
- Standardization: Develop standard operating procedures (SOPs) to eliminate unnecessary steps.
- Value stream mapping: Identify and eliminate non-value-added activities.
- Reduce number of handling of products: Implement the best practices like cross-docking, direct put-away, using right material handling equipment.
- Electronic transaction: Reduce paper and documentation by using hand-held scanners, tabs or PDAs.
Defects
- Error proofing: Implement measures to prevent errors from occurring.
- Quality control: Prevent defects and rework.
- Quality audits: Regularly inspect products and processes.
- Cycle Counting: Do regular cycle counts to reduce inventory errors.
- Employee training: Provide proper training to reduce mistakes.
Underutilized Talent
- Employee involvement: Encourage employee suggestions and ideas.
- Cross-training: Develop employees’ skills and capabilities.
- Job rotation: Provide opportunities for employees to learn new tasks.
5. Implement the Pilot Project
Implement lean initiatives on a small scale to test their effectiveness before widespread adoption. Start with pilot projects in specific areas of the warehouse to evaluate the impact of lean practices. This could involve applying lean tools like 5S (Sort, Set in order, Shine, Standardize, Sustain), Kanban systems, or value stream mapping in a controlled setting. Monitor the outcomes of these pilot projects to identify best practices, potential challenges, and areas needing adjustment. Successful pilots can then be scaled up across the entire operation.
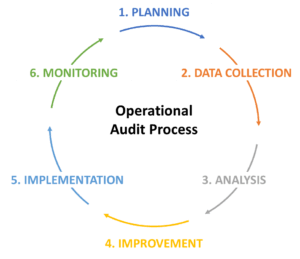
6. Scale-up, Monitor & Improve
Track key performance indicators (KPIs) to assess progress and identify areas for further improvement. KPIs might include metrics like order fulfillment time, inventory turnover, error rates, and space utilization. Regularly review performance data to ensure that lean initiatives are delivering the desired outcomes. Use this information to make data-driven decisions and continuously refine processes. Establish a routine for periodic evaluations and updates to keep lean practices aligned with evolving business needs and market conditions.
 Conclusion
Conclusion
By embracing lean warehousing principles, businesses can create a more efficient, responsive, and profitable warehouse operation. Lean warehousing not only helps in reducing costs and eliminating waste but also fosters a culture of continuous improvement and employee engagement. The result is a streamlined operation that can quickly adapt to changes, meet customer demands more effectively, and contribute to overall business growth and competitiveness.












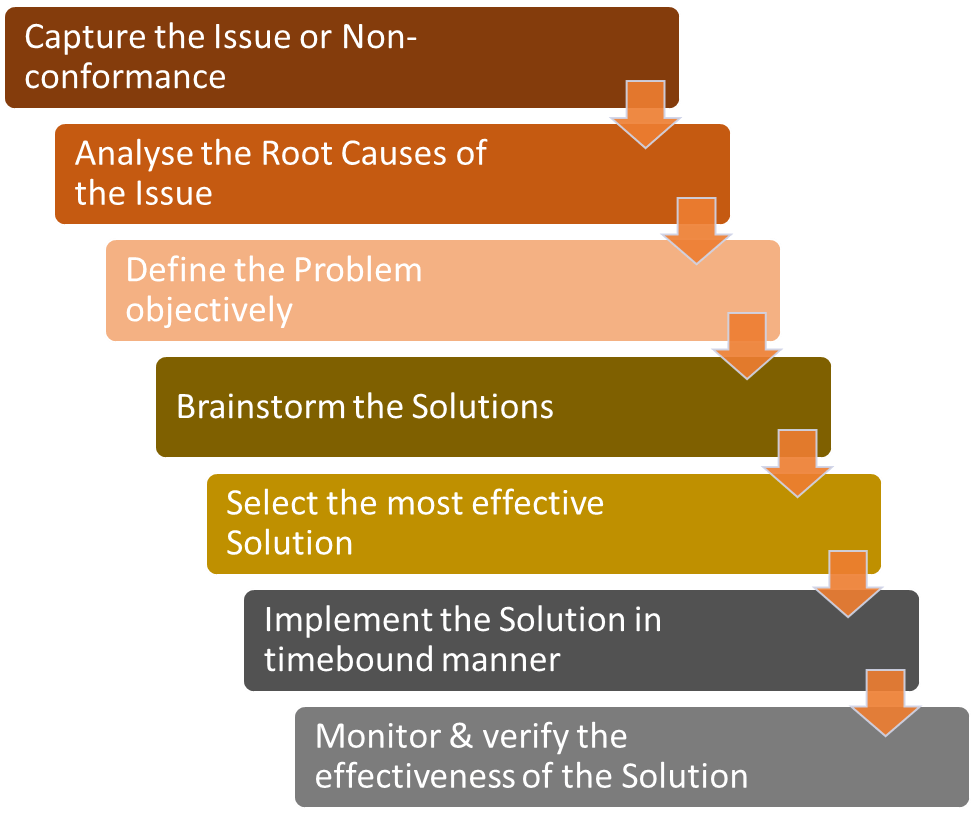 The process involves application of various problem-solving techniques e.g. 5 Whys to define the problem accurately, data analysis and Ishikawa (fishbone) diagram for the root cause analysis, brainstorming etc.
The process involves application of various problem-solving techniques e.g. 5 Whys to define the problem accurately, data analysis and Ishikawa (fishbone) diagram for the root cause analysis, brainstorming etc.
